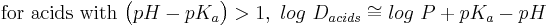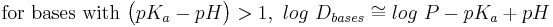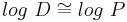Partition coefficient
In chemistry and the pharmaceutical sciences, a partition- (P) or distribution coefficient (D) is the ratio of concentrations of a compound in the two phases of a mixture of two immiscible solvents at equilibrium.[1] The terms "gas/liquid partition coefficient" and "air/water partition coefficient" are sometimes used for dimensionless forms of the Henry's law constant. Hence these coefficients are a measure of differential solubility of the compound between these two solvents. The phrase "Partition Coefficient" is now considered obsolete by IUPAC, and "partition constant," "partition ratio," or "distribution ratio," are all more appropriate terms that should be used.[2]
Normally one of the solvents chosen is water while the second is hydrophobic such as octanol.[3] Hence both the partition and distribution coefficient are measures of how hydrophilic ("water loving") or hydrophobic ("water fearing") a chemical substance is. A partition coefficient can also be used when one or both solvents is a solid. In medical practice, partition coefficients are useful for example in estimating distribution of drugs within the body. Hydrophobic drugs with high partition coefficients are preferentially distributed to hydrophobic compartments such as lipid bilayers of cells while hydrophilic drugs (low partition coefficients) preferentially are found in hydrophilic compartments such as blood serum.
Contents |
Partition coefficient and log P
The partition coefficient is a ratio of concentrations of un-ionized compound between the two solutions. To measure the partition coefficient of ionizable solutes, the pH of the aqueous phase is adjusted such that the predominant form of the compound is un-ionized. The logarithm of the ratio of the concentrations of the un-ionized solute in the solvents is called log P: The log P values is also known as a measure of lipophilicity.
Distribution coefficient and log D
The distribution coefficient is the ratio of the sum of the concentrations of all forms of the compound (ionized plus un-ionized) in each of the two phases. For measurements of distribution coefficient, the pH of the aqueous phase is buffered to a specific value such that the pH is not significantly perturbed by the introduction of the compound. The logarithm of the ratio of the sum of concentrations of the solute's various forms in one solvent, to the sum of the concentrations of its forms in the other solvent is called Log D:
In addition, log D is pH dependent, hence one must specify the pH at which the log D was measured. Of particular interest is the log D at pH = 7.4 (the physiological pH of blood serum).
For un-ionizable compounds, log P = log D at any pH.
Applications
Pharmacology
A drug's distribution coefficient strongly affects how easily the drug can reach its intended target in the body, how strong an effect it will have once it reaches its target, and how long it will remain in the body in an active form.
LogP is one criterion used in medicinal chemistry to assess the druglikeness of a given molecule, and used to calculate lipophilic efficiency, a function of potency and LogP that evaluate the quality of research compounds.[4][5] For a given compound lipophilic efficiency is defined as the pIC50 (or pEC50) of interest minus the LogP of the compound.
Pharmacokinetics
In the context of pharmacokinetics (what the body does to a drug), the distribution coefficient has a strong influence on ADME properties (Absorption, Distribution, Metabolism, and Excretion) of the drug. Hence the hydrophobicity of a compound (as measured by its distribution coefficient) is a major determinant of how drug-like it is. More specifically, for a drug to be orally absorbed, it normally must first pass through lipid bilayers in the intestinal epithelium (a process known as transcellular transport). For efficient transport, the drug must be hydrophobic enough to partition into the lipid bilayer, but not so hydrophobic, that once it is in the bilayer, it will not partition out again.[6] Likewise, hydrophobicity plays a major role in determining where drugs are distributed within the body after absorption and as a consequence in how rapidly they are metabolized and excreted.
Pharmacodynamics
In the context of pharmacodynamics (what a drug does to the body), the hydrophobic effect is the major driving force for the binding of drugs to their receptor targets.[7][8] On the other hand, hydrophobic drugs tend to be more toxic because they, in general, are retained longer, have a wider distribution within the body (e.g., intracellular), are somewhat less selective in their binding to proteins, and finally are often extensively metabolized. In some cases the metabolites may be chemically reactive. Hence it is advisable to make the drug as hydrophilic as possible while it still retains adequate binding affinity to the therapeutic protein target.[9] Therefore the ideal distribution coefficient for a drug is usually intermediate (not too hydrophobic nor too hydrophilic).
Consumer Products
Many other industries take into account distribution coefficients for example in the formulation of make-up, topical ointments, dyes, hair colors and many other consumer products.
Agrochemicals
Hydrophobic insecticides and herbicides tend to be more active. Hydrophobic agrochemicals in general have longer half lives and therefore display increased risk of adverse environmental impact.
Metallurgy
In metallurgy, the partition coefficient is an important factor in determining how different impurities are distributed between molten and solidified metal. It is a critical parameter for purification using zone melting, and determines how effective an impurity can be removed using directional solidification, described by the Scheil equation.
Environmental
The hydrophobicity of a compound can give scientists an indication of how easily a compound might be taken up in groundwater to pollute waterways, and its toxicity to animals and aquatic life.[10] Distribution coefficients may be measured or predicted for compounds currently causing problems or with foresight to gauge the structural modifications necessary to make a compound environmentally more friendly in the research phase.
In the field of hydrogeology, the octanol water partition coefficient, or Kow, is used to predict and model the migration of dissolved hydrophobic organic compounds in soil and groundwater.
Measurement
Shake flask (or tube) method
The classical and most reliable method of log P determination is the shake-flask method, which consists of dissolving some of the solute in question in a volume of octanol and water, then measuring the concentration of the solute in each solvent. The most common method of measuring the distribution of the solute is by UV/VIS spectroscopy. There are a number of pros and cons to this method:
Pros:
-
- Most accurate method
- Accurate for broadest range of solutes (neutral and charged compounds applicable)
- Chemical structure does not have to be known beforehand.
Cons:
-
- Time consuming (>30 minutes per sample)
- Octanol and water must be premixed and equilibrated (takes at least 24 hours to equilibrate)
- Complete solubility must be attained, and it can be difficult to detect small amounts of undissolved material.
- The concentration vs. UV-Vis response must be linear over the solute's concentration range. (See Beer-Lambert law)
- If the compound is extremely lipophilic or hydrophilic, the concentration in one of the phases will be exceedingly small, and thus difficult to quantify.
- Relative to chromatographic methods, large amounts of material are required.
As an alternative to UV/VIS spectroscopy other methods can be used to measure the distribution, one of the best is to use a carrier free radiotracer. In this method (which is well suited for the study of the extraction of metals) a known amount of a radioactive material is added to one of the phases. The two phases are then brought into contact and mixed until equilibrium has been reached. Then the two phases are separated before the radioactivity in each phase is measured. Using an energy dispersive detector (such as a high purity germanium detector) allows the use of several different radioactive metals at once, whereas the simpler gamma ray detectors only allow one radioactive element to be used in the sample.
If the volume of both of the phases are the same then the math is very simple.
For a hypothetical solute (S)
D or P = radioactivity of the organic phase / radioactivity of the aqueous phase
D or P = [Sorganic]/[Saqueous]
In such an experiment using a carrier free radioisotope the solvent loading is very small, hence the results are different from those obtained when the concentration of the solute is very high. A disadvantage of the carrier free radioisotope experiment is that the solute can absorb on the surfaces of the glass (or plastic) equipment or at the interface between the two phases. To guard against this the mass balance should be calculated.
It should be the case that
radioactivity of the organic phase + radioactivity of the aqueous phase = initial radioactivity of the phase bearing the radiotracer
For nonradioactive metals, it is possible in some cases to use ICP-MS or ICP-AES. Sadly ICP methods often suffer from many interferences that do not apply to gamma spectroscopy so hence the use of radio-tracers (counted by gamma ray spectroscopy) is often more straightforward.
HPLC determination
A faster method of log P determination makes use of high-performance liquid chromatography. The log P of a solute can be determined by correlating its retention time with similar compounds with known log P values.[11]
Pros:
-
- Fast method of determination (5-20 minutes per sample)
Cons:
-
- The solute's chemical structure must be known beforehand.
- Since the value of log P is determined by linear regression, several compounds with similar structures must have known log P values.
- Different chemical classes will have different regression parameters, hence extrapolations to other chemical classes (applying a regression equation derived from one chemical class to a second chemical class) are not reliable.
Electrochemical methods
In the recent past some experiments using polarised liquid interfaces have been used to examine the thermodynamics and kinetics of the transfer of charged species from one phase to another. Two main methods exist.
- ITIES, Interfaces between two immiscible electrolyte solutions,[12] which, for example, has been used at Ecole Polytechnique Fédérale de Lausanne. [1]
- Droplet experiments, which have been used by Alan Bond, Frank Marken [2], and also by the team at the Ecole Polytechnique Fédérale de Lausanne. Here a reaction at a triple interface between a conductive solid, droplets of a redox active liquid phase and an electrolyte solution have been used to determine the energy required to transfer a charged species across the interface.[13]
Prediction
QSPR (Quantitative Structure-Property Relationship) algorithms calculate a log P in several different ways:
- Atomic based prediction (atomic contribution; AlogP, XlogP,[14] MlogP, etc.)
- The simplest method for prediction of log P is parameterizing the contributions of various atoms to the over all molecular partition coefficient using constrained least squares fitting to a training set of compounds with experimentally measured partition coefficients.[15][16][17] In order to get reasonable correlations, the most common elements contained in drugs (hydrogen, carbon, oxygen, sulfur, nitrogen, and halogens) are divided into several different atom types depending on the environment of the atom within the molecule. While this method is generally the least accurate, the advantage is that it is the most general, being able to provide at least a rough estimate for a wide variety of molecules.
- Fragment based prediction (group contribution; ClogP, etc.)
- It has been shown that the log P of a compound can be determined by the sum of its non-overlapping molecular fragments (defined as one or more atoms covalently bound to each other within the molecule). Fragmentary log P values have been determined in a statistical method analogous to the atomic methods (least squares fitting to a training set). In addition, Hammett type corrections are included to account of electronic and steric effects. This method in general gives better results than atomic based methods, but cannot be used to predict partition coefficients for molecules containing unusual functional groups for which the method has not yet been parameterized (most likely because of the lack of experimental data for molecules containing such functional groups).[18][19]
- Data mining prediction
- A typical data mining based prediction uses support vector machines,[20] decision trees, or neural networks.[21] This method is usually very successful for calculating log P values when used with compounds that have similar chemical structures and known log P values.
- Molecule mining prediction
- Molecule mining approaches apply a similarity matrix based prediction or an automatic fragmentation scheme into molecular substructures. Furthermore there exist also approaches using maximum common subgraph searches or molecule kernels.
- Estimation of log D (at a given pH) from log P and pKa:[22]
- exact expressions:
- approximations for when the compound is largely ionized:
- approximation when the compound is largely un-ionized:
- Prediction of pKa
- For prediction of pKa, which in turn can be used to estimate log D, Hammett type equations have frequently been applied.[23] See[24] for a recent review of newer methods.
Some Octanol-Water partition coefficient data
The given values[25] are sorted by the partition coefficient. Acetamide is hydrophilic and 2,2',4,4',5-Pentachlorobiphenyl is lipophilic.
| Component | log POW | T (°C) |
|---|---|---|
| Acetamide[26] | -1.16 | 25 |
| Methanol[27] | -0.82 | 19 |
| Formic acid[28] | -0.41 | 25 |
| Diethyl ether[27] | 0.83 | 20 |
| p-Dichlorobenzene[29] | 3.37 | 25 |
| Hexamethylbenzene[29] | 4.61 | 25 |
| 2,2',4,4',5-Pentachlorobiphenyl[30] | 6.41 | Ambient |
Values for other compounds may be found in Sangster Research Laboratories' database.[31]
Limitations
Log P is not an accurate determinant of lipophilicity for ionizable compounds because it only correctly describes the partition coefficient of neutral (uncharged) molecules. Taking the example of drug discovery we see how the limitations of log P can affect research. Since the majority of drugs (approximately 80%) are ionizable, log P is not an appropriate predictor of a compound's behaviour in the changing pH environments of the body. The distribution coefficient (Log D) is the correct descriptor for ionizable systems. Alternatively, use may be made of the apparent partition coefficient, which is defined as follows: (true partition coefficient) x (fraction of the drug that is unionised). Clearly, if the drug is 100% unionised then Papparent = Ptrue.
See also
- Cheminformatics
- ITIES
- Ionic partition diagram
- Chemicalize.org:List of predicted structure based properties
References
- ^ Leo A, Hansch C, and Elkins D (1971). "Partition coefficients and their uses". Chem Rev 71 (6): 525–616. doi:10.1021/cr60274a001.
- ^ Wilkinson, Andrew M.; McNaught, Alan D. (1997). "Partition Coefficient". Compendium of Chemical Terminology: IUPAC Recommendations. Oxford: Blackwell Science. doi:10.1351/goldbook. ISBN 0-86542-684-8. http://goldbook.iupac.org/P04437.html.
- ^ Sangster, James (1997). Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry, Vol. 2 of Wiley Series in Solution Chemistry. Chichester: John Wiley & Sons Ltd.. pp. 178 pages. ISBN 978-0471973973.
- ^ Edwards MP, Price DA (2010). "Role of Physicochemical Properties and Ligand Lipophilicity Efficiency in Addressing Drug Safety Risks". Annual Reports in Medicinal Chemistry. Annual Reports in Medicinal Chemistry 45: 381–391. doi:10.1016/S0065-7743(10)45023-X. ISBN 9780123809025.
- ^ Leeson PD, Springthorpe B (November 2007). "The influence of drug-like concepts on decision-making in medicinal chemistry". Nat Rev Drug Discov 6 (11): 881–90. doi:10.1038/nrd2445. PMID 17971784.
- ^ Kubinyi H (1979). "Nonlinear dependence of biological activity on hydrophobic character: the bilinear model". Farmaco [Sci] 34 (3): 248–76. PMID 43264.
- ^ Eisenberg D, McLachlan AD (1986). "Solvation energy in protein folding and binding". Nature 319 (6050): 199–203. doi:10.1038/319199a0. PMID 3945310.
- ^ Miyamoto S, Kollman PA (1993). "What determines the strength of noncovalent association of ligands to proteins in aqueous solution?". Proc Natl Acad Sci USA 90 (18): 8402–6. doi:10.1073/pnas.90.18.8402. PMC 47364. PMID 8378312. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=47364.
- ^ Pliska, Vladimir; Testa B, Van De Waterbeemd H (1996). Lipophilicity in Drug Action and Toxicology. New York: John Wiley & Sons Ltd.. pp. 439 pages. ISBN 978-3527293834.
- ^ Cronin D, Mark T (2006). "The Role of Hydrophobicity in Toxicity Prediction". Current Computer - Aided Drug Design 2 (4): 405–413. doi:10.2174/157340906778992346.
- ^ Valkó K (2004). "Application of high-performance liquid chromatography based measurements of lipophilicity to model biological distribution". Journal of Chromatography A 1037 (1–2): 299–310. doi:10.1016/j.chroma.2003.10.084. PMID 15214672.
- ^ Ulmeanu SM, Jensen H, Bouchard G, Carrupt PA, Girault HH (2003). "Water-oil partition profiling of ionized drug molecules using cyclic voltammetry and a 96-well microfilter plate system". Pharm. Res. 20 (8): 1317–22. doi:10.1023/A:1025025804196. PMID 12948031.
- ^ Bond AM, Marken F (1994). "Mechanistic aspects of the electron and ion transport processes across the electrode". Journal of Electroanalytical Chemistry 372 (1–2): 125–135. doi:10.1016/0022-0728(93)03257-P.
- ^ Cheng, Tiejun; Yuan Zhao; Xun Li; Fu Lin; Yong Xu; Xinglong Zhang; Yan Li; Renxiao Wang; Luhua Lai (2007). "Computation of Octanol−Water Partition Coefficients by Guiding an Additive Model with Knowledge". Journal of Chemical Information and Modeling 47 (6): 2140–2148. doi:10.1021/ci700257y. PMID 17985865. http://www.sioc-ccbg.ac.cn/?p=42&software=xlogp3.
- ^ Ghose AK, Crippen GM (1986). "Atomic Physicochemical Parameters for Three-Dimensional Structure-Directed Quantitative Structure-Activity Relationships I. Partition Coefficients as a Measure of Hydrophobicity". Journal of Computational Chemistry 7 (4): 565–577. doi:10.1002/jcc.540070419.
- ^ Ghose AK, Viswanadhan VN, Wendoloski, JJ (1998). "Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragmental Methods: An Analysis of AlogP and ClogP Methods". Journal of Physical Chemistry A 102 (21): 3762–3772. doi:10.1021/jp980230o.
- ^ Moriguchi I, Hirono S, Liu Q, Nakagome I, Matsushita Y (1992). "Simple method of calculating octanol/water partition coefficient". Chem Pharm Bull 40 (1): 127–130.
- ^ Hansch, Corwin; Leo A (1979). Substituent Constants for Correlation Analysis in Chemistry and Biology. New York: John Wiley & Sons Ltd.. pp. 178 pages. ISBN 978-0471050629.
- ^ Leo, Albert; Hoekman DH, Hansch C (1995). Exploring QSAR, Hydrophobic, Electronic, and Steric Constants. Washington, DC: American Chemical Society. ISBN 978-0841230606.
- ^ Liao Q, Yao J, Yuan S (2006). "SVM approach for predicting LogP". Mol. Divers. 10 (3): 301–9. doi:10.1007/s11030-006-9036-2. PMID 17031534.
- ^ Molnár L, Keseru GM, Papp A, Gulyás Z, Darvas F (2004). "A neural network based prediction of octanol-water partition coefficients using atomic5 fragmental descriptors". Bioorg. Med. Chem. Lett. 14 (4): 851–3. doi:10.1016/j.bmcl.2003.12.024. PMID 15012980.
- ^ Scherrer RA, Howard SM (1977). "Use of distribution coefficients in quantitative structure-activity relationships". J Med Chem 20 (1): 53–8. doi:10.1021/jm00211a010. PMID 13215.
- ^ Perrin, DD; Dempsey B, Serjeant EP (1981). pKa Prediction for Organic Acids and Bases. London: Chapman & Hall. ISBN 041222190X.
- ^ Fraczkiewicz, R (2007). "In Silico Prediction of Ionization". In Testa B and van de Waterbeemd H, eds.. Comprehensive Medicinal Chemistry II. vol. 5. Amsterdam, The Netherlands: Elsevier.
- ^ Dortmund Data Bank
- ^ Wolfenden R (1978). "Interaction of the peptide bond with solvent water: a vapor phase analysis". Biochemistry 17 (1): 201–4. doi:10.1021/bi00594a030. PMID 618544.
- ^ a b Collander R; Lindholm, Martta; Haug, Carl Monthei; Stene, JöRgine; Sörensen, Nils Andreas (1951). "The partition of organic compounds. between higher alcohols and water". Acta Chem Scand 5: 774–780. doi:10.3891/acta.chem.scand.05-0774.
- ^ Whitehead KE, Geankoplis CJ (1955). "Separation of Formic and Sulfuric Acids by Extraction". Ind Eng Chem 47 (10): 2114–2122. doi:10.1021/ie50550a029.
- ^ a b Wasik SP, Tewari YB, Miller MM, Martire DE (1981). "Octanol - Water Partition Coefficients and Aqueous Solubilities of Organic Compounds". NBS Techn Rep 81 (2406): S1–56.
- ^ Brodsky J, Ballschmiter K (1988). "Reversed phase liquid chromatography of PCBs as a basis for calculation of water solubility and Kow for polychlorobiphenyls". Fresenius Z Anal Chem 331 (3–4): 295–301. doi:10.1007/BF00481899.
- ^ Sangster J. "LOGKOW: A databank of evaluated octanol-water partition coefficients (LogP)". Sangster Research Laboratories. http://logkow.cisti.nrc.ca/logkow/. Retrieved 2010-04-04.
External links
There are many logP calculators or predictors available both commercially and for free.
- Chemistry Development Kit
- JOELib
- BioByte ClogP/Bio-Loom
- ACD/LogP DB a commercial application that calculates LogP values and includes the largest commercially available database of experimental logP values with calculation of Rule-of-5 parameters
- ACD/LogP Freeware Download the free logP calculator
- Simulations Plus - S+logP an application for calculating logP with high accuracy[1]
- ALOGPS Free online calculations and comparison of 10 logP methods
- Molecular Property Explorer
- Free online logP calculations using ChemAxon's Marvin and Calculator Plugins - requires Java]
- miLogP free logP and Rule of Five calculator by Molinspiration
- an overview of on-line WWW resources for logP and other PhysProp calculations
- PreADMET Web-based logP/logS and ADME/Tox prediction program
- XLOGP3 an online and standalone logP calculator (including rule-of-5). Free for academy.
- ^ Tetko, IV; Poda GI (2007). "Property-based logP prediction". In Mannhold R. Molecular Drug Properties: Measurement and Prediction. Weinheim, Germany: Wiley-VCH.
|
||||||||||||||
![log\ P_{oct/wat} = log\Bigg(\frac{\big[solute\big]_{octanol}}{\big[solute\big]_{water}^{un-ionized}}\Bigg)](/2012-wikipedia_en_all_nopic_01_2012/I/8d20065895fcd23c2f7320352432290e.png)
![log\ D_{oct/wat} = log\Bigg(\frac{\big[solute\big]_{octanol}}{\big[solute\big]_{water}^{ionized}%2B\big[solute\big]_{water}^{neutral}}\Bigg)](/2012-wikipedia_en_all_nopic_01_2012/I/d4dd37790cb0df9ea95d47882af65fac.png)
![log\ D_{acids} = log\ P %2B log\Bigg[\frac{1}{(1%2B10^{pH-pK_a})}\Bigg]](/2012-wikipedia_en_all_nopic_01_2012/I/1d271a15d9e5bfcea818b489906b44c7.png)
![log\ D_{bases} = log\ P %2B log\Bigg[\frac{1}{(1%2B10^{pK_a-pH})}\Bigg]](/2012-wikipedia_en_all_nopic_01_2012/I/70b14db31996c707a40236716853f0c9.png)